The mechanism of ammonium bisulfate formation and decomposition over V/WTi catalysts for NH3-selective catalytic reduction at various temperatures†
Abstract
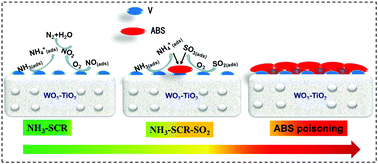
In this study, the mechanism of ammonium bisulfate (ABS) formation and decomposition over V/WTi for the NH3-selective catalytic reduction (SCR) at various temperatures was deeply investigated. Bridged bidentate, chelating bidentate, and tridentate sulfates bound to TiO2 were formed as dominant intermediates at 200, 250, and 300 °C, respectively. These sulfates reacted with affinitive ammonium species to form ammonium (bi)sulfate species and also covered the active sites and embedded the VOSO4 intermediates, which resulted in an inferior intrinsic NH3-SCR conversion rate at 200 °C and 250 °C. At 300 °C, trace amounts of ABS on TiO2 presented no influence on the NH3-SCR performance. The electrons deviating towards sulfates through the bond between ABS and metal oxides (WO3 and TiO2) weakened the stability of ABS and lowered its decomposition temperature, whereas the vanadia species played the opposite role due to the sulfur species existing in an electron saturation state with the formation of the VOSO4 intermediate. The presence of NO + O2 could break the bonds inside ABS and it could react with the ammonium species originating from ABS, which pulls NH3 out of the ABS formation equilibrium and accelerates its decomposition and competitively inhibits its formation. Correspondingly, the faster NH3-SCR conversion rate and higher N2 selectivity improve the ABS poisoning resistance of the V/WTi catalyst at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...