Polar metal–formate frameworks templated with 1,2-diaminoethane–water assemblies showing ferromagnetic and ferroelectric properties†
Abstract
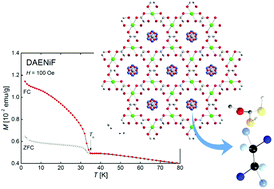
A set of five novel formate frameworks templated with assemblies comprising diprotonated 1,2-diaminoethane (DAE) and a water molecule of the formula: [NH3(CH2)2NH3]M2(HCOO)6·H2O, where M = Mg, Mn, Co, Ni, Zn, has been synthesized. Four compounds crystallize in the polar R3 space group and one in the chiral P6322 space group (Ni-analog) at room temperature. The polyammonium–water assemblies, mutually joined by hydrogen bonds, fill the cavities of the frameworks and are disordered in the three latter compounds. Additional disorder is found in the Ni-sample as the DAE2+–H2O couple is placed in a special position on the 63 screw axis. IR spectroscopy provides evidence of proton dynamic disorder within the assemblies, which turns into a static one at low temperatures. The crystals preserve their arrangement up to approximately 370 K as shown by differential calorimetric measurements and temperature-dependent IR spectroscopy. The ferroelectric nature of a representative of the family, DAEMgF, at room temperature has been confirmed by pyroelectric measurements. It has been found that the spontaneous polarization may be changed by an external electric field. The magnetic studies reveal a weak ferromagnetic behavior within 8.5–35 K for magnetically active ions: Mn, Co, and Ni.


 Please wait while we load your content...
Please wait while we load your content...