4N electron aromatic cycles in polycyclic hydrocarbons†
Abstract
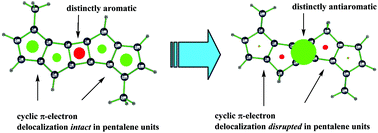
Polycyclic fully conjugated hydrocarbons in which aromatics are fused to aromatics – or aromatics to antiaromatics – are important as potential organic semiconductors. Herein we explore the only remaining fusion pattern of antiaromatics to antiaromatics. It is shown computationally that the central antiaromatic unit (cyclobutadiene or pentalene) in such a three-unit polycyclic hydrocarbon, generated by fusion of three antiaromatic molecules, turns aromatic according to magnetic shielding (NICS) criteria. The resulting neutral 4N electron molecules possess a 4N π electron perimeter with pronounced CC bond length equalization (as indicated by the HOMA geometric index) and significant aromatic stabilization energies (computed using the isomerization-stabilization method) and could be promising synthetic targets with small HOMO–LUMO gaps.


 Please wait while we load your content...
Please wait while we load your content...