Dissection of H-bonding interactions in a glycolic acid–water dimer
Abstract
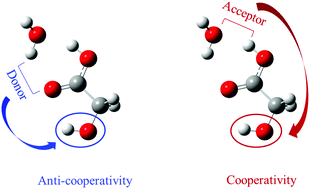
The binding strength and collective effects of multiple H-bonds in the glycolic acid–water dimer were studied in comparison to the aromatic analog, 9-hydroxy-9-fluorene carboxylic acid (9HFCA). Quantitative analysis by the generalized Kohn–Sham energy decomposition analysis shows that the energy difference in each specific physical interaction, from a glycolic acid–water dimer to a 9HFCA–water dimer, is small and amounts to less than 5% of the binding energy of the 9HFCA–water dimer. Extensive comparison of further, similar H-bonded complexes with widely varying binding strengths reinforces their excellent analogy in that the fluorene group acts as a non-interfering spectator for intermolecular H-bonding interactions. With reference to the spectroscopic measurement on the 9HFCA–water dimer (8.51 ± 0.09 kcal mol−1), the binding energy of the glycolic acid–water dimer is estimated to be 8.51 ± 0.31 kcal mol−1, a much better accuracy than previous reports. Furthermore, correlating the infrared spectra of 9HFCA H-bonded complexes provides a circumstantial probing of the existence and consequences of cooperative and anti-cooperative behaviors in the glycolic acid–water dimer. Our studies point to the interesting H-bonding phenomena in the glycolic acid–water dimer, which may inspire challenging experiments in future.


 Please wait while we load your content...
Please wait while we load your content...