Controlling the kinetic and thermodynamic stability of cationic clusters by the addition of molecules or counterions
Abstract
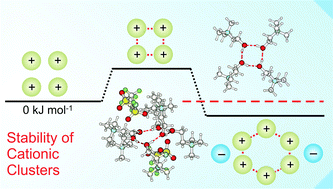
Whereas ion pairing is one of the most fundamental atomic interactions in chemistry and biology, pairing between like-charged ions remains an elusive concept. This phenomenon was only reported for large-scaled structures, assemblies or stabilizing frameworks. Recently, we could report the formation of cationic clusters in pure ionic liquids. In such structures like-charge repulsion is attenuated by cooperative hydrogen bonds. In the present work, we investigate the possible formation of cationic clusters in the gas phase beyond those found in the neutral ionic liquids wherein the positive charges are fully balanced by anions. Based on the ionic liquid (2-hydroxyethyl)trimethylammonium bis(trifluoromethylsulfonyl)imide we calculated differently charged cationic clusters including varying numbers of cation-like molecules (3-3-dimethyl-1-butanol) or ionic liquid anions (NTf2). We give the number of molecules or anions which are needed to transfer the cationic clusters from the meta-stable into the thermodynamically stable regime. We analyze the charge, the size and the structural motif of these clusters. A particular focus we put on the cooperativity of hydrogen bonding and the role of dispersion forces for the cluster stability. We also show that interaction energies and charge transfer within the cationic clusters can be related to spectroscopic parameters such as NMR chemical shifts and IR vibrational frequencies. Finally, we suggest clusters which should be observable in demanding gas phase experiments.


 Please wait while we load your content...
Please wait while we load your content...