A novel self-activation mechanism of Candida antarctica lipase B†
Abstract
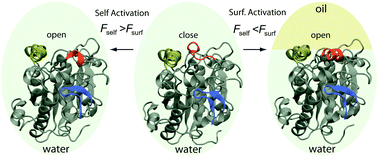
Candida antarctica lipase B (CalB), resembling many other lipases structure-wise, contains a flexible lid that undergoes a surprisingly large conformational change when catalyzing hydrophobic substrates (e.g. triglycerides). Despite extensive and important applications in industry, it is so far still elusive whether CalB can be activated on a hydrophobic surface, like other lipases. From large-scale all-atom molecular dynamics simulations, we discovered an open state that strikingly shows a much wider and more stable entrance to the catalytic site than the one suggested by previous crystal structures. Simulations demonstrate that in the newly found open state CalB possesses a “lid-holder” structure that intimately harbors the lid of CalB, i.e. a remarkable self-activation mechanism. To account for the unusual interfacial activation of CALB revealed in a recent experiment, we further introduce a simple model: the activation occurs only when the binding free energy between the lid and a hydrophobic surface is larger than a critical value, 4.0 kcal mol−1 that is the one between the lid and the “lid-holder”. Our findings shed light on possible protein engineering of lipases to permit either self-activation with broadened catalytic targets (including water soluble ones) or surface activation with elevated activities.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...