A systematic study of the influence of mesoscale structuring on the kinetics of a chemical reaction†
Abstract
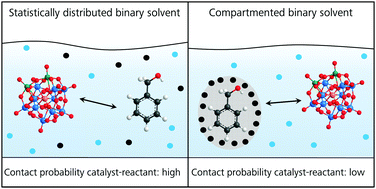
In this contribution, we (i) link the mesoscopic structuring of the binary structured solvent mixture H2O/tert-butanol (TBA) to the kinetics and the efficacy of the oxidation of benzyl alcohol (BA) to the corresponding aldehyde catalyzed by H5PMo10V2O40. We also compare the catalytic efficacy of this reaction in the mesoscopically structured solvent H2O/TBA to an unstructured (or very weakly structured) solvent H2O/ethanol (EtOH). In this context, we (ii) also give a methodological outline on how to study systematically the catalytic efficacy of chemical reactions as a function of the mesoscale structuring of a binary solvent. We demonstrate that the obtained yields of benzyl aldehyde depend on the type of mesoscopic structuring of the binary solvent H2O/TBA. An elevated catalytic performance of at least 100% is found for unstructured binary mixtures H2O/TBA compared to compartmented binary mixtures H2O/TBA. We conclude that compartmentation of both the organic substrate and the catalyst in TBA and water-rich micro phases seems to be unfavorable for the catalytic efficacy.
- This article is part of the themed collections: 2017 PCCP HOT Articles and Surface chemistry and interface science


 Please wait while we load your content...
Please wait while we load your content...