Breakdown of the Stokes–Einstein water transport through narrow hydrophobic nanotubes
Abstract
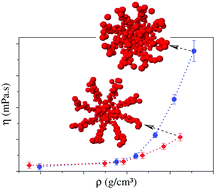
In this paper the transport properties of water confined inside hydrophobic and hydrophilic nanotubes are compared for different nanotube radii and densities. While for wider nanotubes the nature of the wall plays no relevant role in the water mobility, for small nanotubes the hydrophobic confinement presents a peculiar behavior. As the density is increased the viscosity shows a huge increase associated with a small increase in the diffusion coefficient. This breakdown in the Stokes–Einstein relation for diffusion and viscosity was observed in the hydrophobic, but not in the hydrophilic nanotubes. The mechanism underlying this behavior is explained in terms of the structure of water under confinement. This result indicates that some of the features observed for water inside hydrophobic carbon nanotubes cannot be observed in other nanopores.


 Please wait while we load your content...
Please wait while we load your content...