Monovalent and bivalent cations exchange isotherms for faujasites X and Y†
Abstract
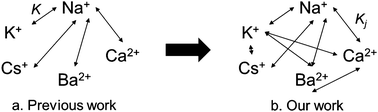
This study addresses the modeling of exchange isotherms for faujasite-type zeolites X and Y with K+, Cs+, Ca2+ and Ba2+ cations based on a large experimental dataset obtained under operating conditions of 0.5 N total normality and an exchange temperature of 80 °C. The isotherm models are based on the mass action law. Ideal solution phase is assumed. Heterogeneity of the solid phase is taken into account by using Barrer and Klinowski's approach to multi-site exchange. Three types of exchange sites are identified on these zeolites. To each exchange site j corresponds a fitted selectivity coefficient Kj. These parameters, estimated by least square method, evaluate the affinity of the studied cations for the identified exchange site. Globally, these fitted coefficients show that the cations considered present better affinity than Na+, especially for type III sites in faujasite X and type II sites in faujasite Y. For bivalent cations, an exchange with Ba2+ is always more favorable than with Ca2+. On faujasite X, type II sites are more strongly preferred by monovalent cations (with the exception of Cs+) than by bivalent ones. The opposite trend is observed on faujasite Y, even for Cs+. These conclusions have been confirmed and are supported by bibliographic data.


 Please wait while we load your content...
Please wait while we load your content...