Li-ion diffusion in Li intercalated graphite C6Li and C12Li probed by μ+SR
Abstract
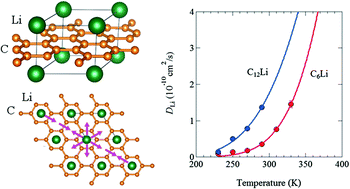
In order to study a diffusive behavior of Li+ in Li intercalated graphites, we have measured muon spin relaxation (μ+SR) spectra for C6Li and C12Li synthesized with an electrochemical reaction between Li and graphite in a Li-ion battery. For both compounds, it was found that Li+ ions start to diffuse above 230 K and the diffusive behavior obeys a thermal activation process. The activation energy (Ea) for C6Li is obtained as 270(5) meV, while Ea = 170(20) meV for C12Li. Assuming a jump diffusion of Li+ in the Li layer of C6Li and C12Li, a self-diffusion coefficient DLi at 310 K was estimated as 7.6(3) × 10−11 (cm2 s−1) in C6Li and 14.6(4) × 10−11 (cm2 s−1) in C12Li.


 Please wait while we load your content...
Please wait while we load your content...