Chiroptical properties of cryptophane-111†
Abstract
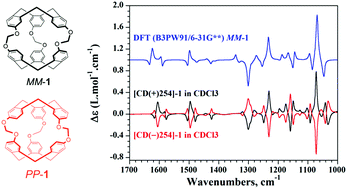
The two enantiomers of cryptophane-111 (1), which possesses the most simplified chemical structure of cryptophane derivatives and exhibits the highest binding constant for xenon encapsulation in organic solution, were separated by HPLC using chiral stationary phases. The chiroptical properties of [CD(+)254]-1 and [CD(−)254]-1 were determined in CH2Cl2 and CHCl3 solutions by polarimetry, electronic circular dichroism (ECD), vibrational circular dichroism (VCD), and Raman optical activity (ROA) experiments and were compared to those of cryptophane-222 (2) derivative. Synchroton Radiation Circular Dichroism (SRCD) spectra were also recorded for the two enantiomers of 1 to investigate low-lying excited states in the 1Bb region. Time-dependent density functional theory (TDDFT) calculations of the ECD and SRCD as well as DFT calculations of the VCD and ROA allowed the [CD(−)254]-PP-1 and [CD(+)254]-MM-1 absolute configurations for 1 in CH2Cl2 and CHCl3 solutions. Similar configurations were found in the solid state from X-ray crystals of the two enantiomers but the chemical structures are significantly different from the one calculated in solution. In addition, the chiroptical properties of the two enantiomers of 1 were independent of the nature of the solvent, which is significantly different to that observed for cryptophane-222 compound. The lack of solvent molecule (CH2Cl2 or CHCl3) within the cavity of 1 can explain this different behaviour between 1 and 2. Finally, we show in this article that the encapsulation of xenon by 1 can be evidenced by ROA following the symmetric breathing mode of the cryptophane-111 skeleton at 150 cm−1.


 Please wait while we load your content...
Please wait while we load your content...