Light-induced antibiotic release from a coumarin-caged compound on the ultrafast timescale†
Abstract
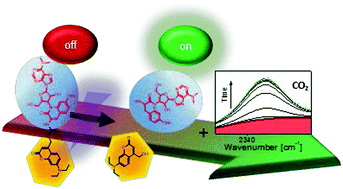
A synthesis route for puromycin caged with the photo-responsive 7-diethylaminocoumarinyl protecting group carbamate was developed. The inactivation and recovery of the cytotoxic effect of puromycin was tested with a XTT cell viability assay. The uncaging mechanism was studied by ultrafast transient absorption spectroscopy and by time-correlated single photon counting. The combination of these results with quantum-chemical calculations provided detailed insights in dynamics upon excitation. Interestingly, a change of the dipole moment due to structural rearrangements of the amino moiety led to an intermolecular charge transfer on the picosecond time-scale. IR measurements marked the successful uncaging via the release of CO2, resulting from the carbamate linker. This decarboxylation constituted the rate-limiting step of the uncaging reaction and occurred on the subsecond timescale. DEACM-puromycin, thus, represents an efficient photo-activatable antibiotic for in-cell applications.


 Please wait while we load your content...
Please wait while we load your content...