Vaporization of protic ionic liquids derived from organic superbases and short carboxylic acids†
Abstract
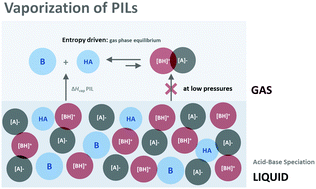
This work presents a comprehensive evaluation of the phase behaviour and cohesive enthalpy of protic ionic liquids (PILs) composed of 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) organic superbases with short-chain length (acetic, propionic and butyric) carboxylic acids. Glass transition temperatures, Tg, and enthalpies of vaporization, ΔHvap, were measured for six [BH][A] (1 : 1) PILs (B = DBN, DBU; A = MeCOO, EtCOO, nPrCOO), revealing more significant changes upon increasing the number of –CH2– groups in the base than in the acid. The magnitude of ΔHvap evidences that liquid PILs have a high proportion of ions, although the results also indicate that in DBN PILs the concentration of neutral species is not negligible. In the gas phase, these PILs exist as a distribution of ion pairs and isolated neutral species, with speciation being dependent on the temperature and pressure conditions – at high temperatures and low pressures the separated neutral species dominate. The higher Tg and ΔHvap of the DBU PILs are explained by the stronger basicity of DBU (as supported by NMR and computational calculations), which increases the extent of proton exchange and the ionic character of the corresponding PILs, resulting in stronger intermolecular interactions in condensed phases.


 Please wait while we load your content...
Please wait while we load your content...