The atmospheric oxidation of CH3OOH by the OH radical: the effect of water vapor†
Abstract
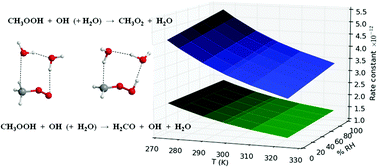
The atmospheric oxidation of methyl hydroperoxide by the hydroxyl radical has been investigated employing high level theoretical methods. This reaction is important in the chemistry of the troposphere because these species contribute to the oxidizing capacity of the atmosphere and therefore we have studied the bare reaction and the effect of the relative humidity as well. In both cases the reaction can proceed either by abstraction of the terminal hydrogen atom of the OH group, producing CH3O2 + H2O, or by abstraction of one hydrogen atom of the CH3 group, forming H2CO + OH + H2O. We have employed BH&HLYP, QCISD and CCSD(T) theoretical methods along with 6-311+G(2df,2p), aug-cc-pVTZ, aug-cc-pVQZ and CBS basis sets to investigate the reaction mechanism, and conventional and variational transition state theory to study the kinetics of the reaction. For the bare reaction we have computed at room temperature, a rate constant of 3.59 × 10−12 cm3 molecule−1 s−1 for the formation of CH3O2 + H2O and of 1.68 × 10−12 cm3 molecule−1 s−1 for the production of H2CO + OH + H2O, with branching ratios of 68% and 32% respectively. Water vapor enhances the rate constant for the formation of CH3O2 + H2O between 2 and 19%, depending on the temperature and relative humidity, whereas the rate constant for the production of H2CO + OH + H2O is enhanced between 0.3 and 5% by the effect of water vapor under the same conditions, which means that the branching ratio for the formation of CH3O2 + H2O is increased up to 2.5%.


 Please wait while we load your content...
Please wait while we load your content...