Crystal chemistry of Mg substitution in NaMnPO4 olivine: concentration limit and cation distribution†
Abstract
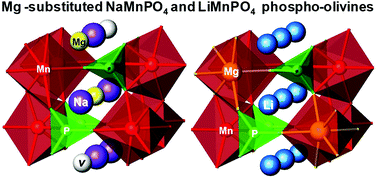
Metal ion substitution in phospho-olivines is an effective way to improve their performance as electrode materials in lithium ion and alternative sodium ion batteries. In this contribution, we examine in detail the crystal structure of Mg-substituted NaMnPO4. The preferential occupancy of the alkaline M1 position by Mg2+ ions has been found for the first time – a phenomenon which appears to be opposite to the case of Mg-substituted LiMnPO4, where Mg2+ and Mn2+ reside in the M2 position. Mg solubility in NaMnPO4 is limited in the range of 0.10 < Mg/(Mg + Mn) < 0.15 mole part. Mg-substituted NaMnPO4 is prepared at 200 °C by ionic exchange reactions involving the participation of mixed dittmarite salts, KMn1−xMgxPO4·H2O. The structural aspects of Mg substitution in NaMnPO4 are studied by combination of powder X-ray diffraction using the Rietveld analysis with IR and electron paramagnetic resonance spectroscopy. The morphologies of precursors and target olivines are examined by means of SEM and EDS. In order to understand the crystal chemistry of Mg-substituted NaMnPO4, we use solid solutions between LiMnPO4 and LiMgPO4 as references. The reference compositions of LiMn1−xMgxPO4 are prepared using the same KMn1−xMgxPO4·H2O precursors as in the case of Mg-substituted NaMnPO4.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...