Magnesia-stabilised zirconia solid electrolyte assisted electrochemical investigation of iron ions in a SiO2–CaO–MgO–Al2O3 molten slag at 1723 K
Abstract
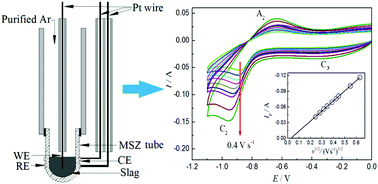
Production of metallic iron through molten oxide electrolysis using inert electrodes is an alternative route for fast ironmaking without CO2 emissions. The fact that many inorganic oxides melt at ultrahigh temperatures (>1500 K) challenges conventional electro-analytical techniques used in aqueous, organic and molten salt electrolytes. However, in order to design a feasible and effective electrolytic process, it is necessary to best understand the electrochemical properties of iron ions in molten oxide electrolytes. In this work, a magnesia-stabilised zirconia (MSZ) tube with a closed end was used to construct an integrated three-electrode cell with a “MSZ|Pt|O2 (air)” assembly functioning as the solid electrolyte, the reference electrode and also the counter electrode. Electrochemical reduction of iron ions was systematically investigated on an iridium (Ir) wire working electrode in a SiO2–CaO–MgO–Al2O3 molten slag at 1723 K by cyclic voltammetry (CV), square wave voltammetry (SWV), chronopotentiometry (CP) and potentiostatic electrolysis (PE). The results show that the electroreduction of the Fe2+ ion to Fe on the Ir electrode in the molten slag follows a single two-electron transfer step, and the rate of the process is diffusion controlled. The peak current on the obtained CVs is proportional to the concentration of the Fe2+ ion in the molten slag and the square root of scan rate. The diffusion coefficient of Fe2+ ions in the molten slag containing 5 wt% FeO at 1723 K was derived to be (3.43 ± 0.06) × 10−6 cm2 s−1 from CP analysis. However, a couple of subsequent processes, i.e. alloy formation on the Ir electrode surface and interdiffusion, were found to affect the kinetics of iron deposition. An ECC mechanism is proposed to account for the CV observations. The findings from this work confirm that zirconia-based solid electrolytes can play an important role in electrochemical fundamental research in high temperature molten slag electrolytes.


 Please wait while we load your content...
Please wait while we load your content...