Colloid–oil–water-interface interactions in the presence of multiple salts: charge regulation and dynamics†‡
Abstract
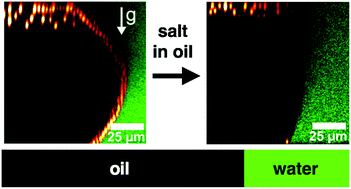
We theoretically and experimentally investigate colloid–oil–water-interface interactions of charged, sterically stabilized, poly(methyl-methacrylate) colloidal particles dispersed in a low-polar oil (dielectric constant ε = 5–10) that is in contact with an adjacent water phase. In this model system, the colloidal particles cannot penetrate the oil–water interface due to repulsive van der Waals forces with the interface whereas the multiple salts that are dissolved in the oil are free to partition into the water phase. The sign and magnitude of the Donnan potential and/or the particle charge is affected by these salt concentrations such that the effective interaction potential can be highly tuned. Both the equilibrium effective colloid–interface interactions and the ion dynamics are explored within a Poisson–Nernst–Planck theory, and compared to experimental observations.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...