Cobalt-porphine catalyzed CO2 electro-reduction: a novel protonation mechanism†
Abstract
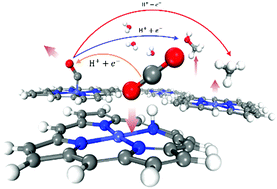
The urgent need for artificially fixing CO2 calls for catalysts of high efficiency. The transition metal functionalized porphyrin (TMP) is one of the most important types of organic catalysts for CO2 reduction. However, the catalytic mechanisms of TMP in CO2 reduction still remain controversial. Starting from the previously neglected catalyst self-protonation model, we uncover a new CO2 reduction mechanism on cobalt-porphine, which involves an indirect proton transfer step occurring at the beginning of the reduction cycle. Based on this protonation mechanism, we demonstrate the different correlations between producing rate and pH for the formation of CO and methane, in good agreement with available experimental observations. Our results reveal how pH and potential affect the CO2 reduction process, providing important clues and insights for further optimization of TMP catalysts.


 Please wait while we load your content...
Please wait while we load your content...