The covalently bound diazo group as an infrared probe for hydrogen bonding environments†
Abstract
Covalently bound diazo groups are frequently found in biomolecular substrates. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N asymmetric stretching vibration (νas) of the diazo group has a large extinction coefficient and appears in an uncongested spectral region. To evaluate the solvatochromism of the C
N asymmetric stretching vibration (νas) of the diazo group has a large extinction coefficient and appears in an uncongested spectral region. To evaluate the solvatochromism of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band for studying biomolecules, we recorded the infrared (IR) spectra of a diazo model compound, 2-diazo-3-oxo-butyric acid ethyl ester, in different solvents. The width of the C
N νas band for studying biomolecules, we recorded the infrared (IR) spectra of a diazo model compound, 2-diazo-3-oxo-butyric acid ethyl ester, in different solvents. The width of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
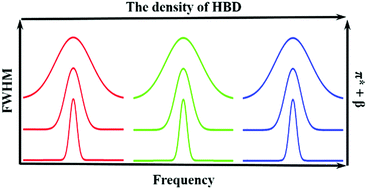
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band was linearly dependent on the Kamlet–Taft solvent parameter, which reflects the polarizability and hydrogen bond accepting ability of the solvent. Therefore, the width of the C
N νas band was linearly dependent on the Kamlet–Taft solvent parameter, which reflects the polarizability and hydrogen bond accepting ability of the solvent. Therefore, the width of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band could be used to probe these properties for a solvent. We found that the position of the C
N νas band could be used to probe these properties for a solvent. We found that the position of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band was linearly correlated with the density of hydrogen bond donor groups in the solvent. We studied the relaxation dynamics and spectral diffusion of the C
N νas band was linearly correlated with the density of hydrogen bond donor groups in the solvent. We studied the relaxation dynamics and spectral diffusion of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band of a natural amino acid, 6-diazo-5-oxo-L-norleucine, in water using nonlinear IR spectroscopy. The relaxation and spectral diffusion time constants of the C
N νas band of a natural amino acid, 6-diazo-5-oxo-L-norleucine, in water using nonlinear IR spectroscopy. The relaxation and spectral diffusion time constants of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band were similar to those of the N
N νas band were similar to those of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band. We concluded that the position and width of the C
N νas band. We concluded that the position and width of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N νas band of the diazo group could be used to probe the hydrogen bond donating and accepting ability of a solvent, respectively. These results suggest that the diazo group could be used as a site-specific IR probe for the local hydration environments.
N νas band of the diazo group could be used to probe the hydrogen bond donating and accepting ability of a solvent, respectively. These results suggest that the diazo group could be used as a site-specific IR probe for the local hydration environments.


 Please wait while we load your content...
Please wait while we load your content...