Insight into thiophene hydrodesulfurization on clean and S-modified MoP(010): a periodic density functional theory study†
Abstract
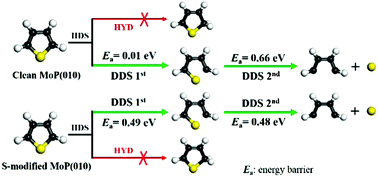
The hydrodesulfurization (HDS) of thiophene on clean and S-modified MoP(010) is investigated to understand the HDS mechanism as well as the surface sulfur (S) atom effect using periodic density functional theory (DFT). The results show that thiophene prefers strongly flat adsorption on both the clean and S-modified surfaces, in either the molecular state or the dissociative state breaking simultaneously one C–S bond, and the adsorption of thiophene can be slightly weakened by the surface S atom. Thermodynamic and kinetic analysis indicates that the HDS of thiophene in both the molecular and dissociative adsorption states prefers to take place along the direct desulfurization (DDS) pathway rather than hydrogenation on both the clean and S-modified MoP(010) surfaces. Surface S shows a promotion effect on the HDS catalytic activity of MoP(010), because the energy barrier/rate constant of the rate-determining step on the DDS pathway is decreased/enlarged under the S modification. Compared with the situation of MoP(001), MoP(010) should have relatively low HDS activity, since a higher energy barrier as well as weaker exothermicity is involved in the reaction on the latter surface.


 Please wait while we load your content...
Please wait while we load your content...