Phase transformations, anisotropic pyroelectric energy harvesting and electrocaloric properties of (Pb,La)(Zr,Sn,Ti)O3 single crystals†
Abstract
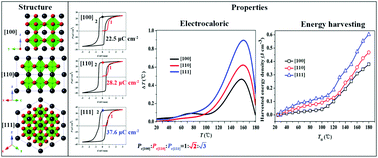
(Pb,La)(Zr,Sn,Ti)O3 (PLZST) single crystals with their chemical composition located at the tetragonal antiferroelectric region are grown via the flux method in a PbO–PbF2–B2O3 mixture. Segregation of the Ti4+ component in the as-grown crystals is observed due to the strong affinity between the oxygen anion and Ti4+ ions. The critical electric field of the antiferroelectric to ferroelectric phase transition is determined to be about 0.5 kV mm−1. The electric field induced ferroelectric phase transforms back into the antiferroelectric phase at a depolarization temperature of 125 °C. Anisotropy of the harvested energy density and electrocaloric behaviors are achieved for the [100], [110] and [111]-oriented PLZST crystals. Based on the thermodynamic theory approach, all the abovementioned behaviors originate from the anisotropic total entropy change. Enhanced electrocaloric strength (0.3 K mm kV−1) and the harvested energy density of 0.62 J cm−3 are obtained in the [111]-oriented PLZST crystals. Our results demonstrate the competence of PLZST single crystals for cooling devices and pyroelectric energy harvesting and provide new opportunities to improve energy harvesting density and electrocaloric properties via the anisotropic structural layout, which make the PLZST crystals attractive for solid state cooling devices and energy conversion technologies.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...