Molecular origin of urea driven hydrophobic polymer collapse and unfolding depending on side chain chemistry†
Abstract
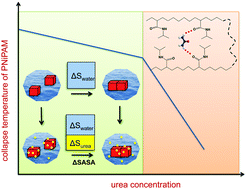
Osmolytes affect hydrophobic collapse and protein folding equilibria. The underlying mechanisms are, however, not well understood. We report large-scale conformational sampling of two hydrophobic polymers with secondary and tertiary amide side chains using extensive molecular dynamics simulations. The calculated free energy of unfolding increases with urea for the secondary amide, yet decreases for the tertiary amide, in agreement with experiment. The underlying mechanism is rooted in opposing entropic driving forces: while urea screens the hydrophobic macromolecular interface and drives unfolding of the tertiary amide, urea's concomitant loss in configurational entropy drives collapse of the secondary amide. Only at sufficiently high urea concentrations bivalent urea hydrogen bonding interactions with the secondary amide lead to further stabilisation of its collapsed state. The observations provide a new angle on the interplay between side chain chemistry, urea hydrogen bonding, and the role of urea in attenuating or strengthening the hydrophobic effect.


 Please wait while we load your content...
Please wait while we load your content...