Isomerisation of an intramolecular hydrogen-bonded photoswitch: protonated azobis(2-imidazole)†
Abstract
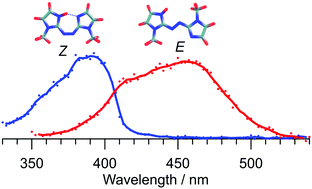
Photoisomerisation of protonated azobis(2-imidazole), an intramolecular hydrogen-bonded azoheteroarene photoswitch molecule, is investigated in the gas phase using tandem ion mobility mass spectrometry. The E and Z isomers exhibit distinct spectral responses, with E–Z photoisomerisation occurring over the 360–520 nm range (peak at 460 nm), and Z–E photoisomerisation taking place over the 320–420 nm range (peak at 390 nm). A minor photodissociation channel involving loss of N2 is observed for the E-isomer with a maximum efficiency at 390 nm, blue-shifted by ≈70 nm relative to the wavelength for maximum photoisomerisation response. Loss of N2 is also the predominant collision-induced dissociation channel. Electronic structure calculations suggest that E-isomer photoisomerisation involves S1(ππ*) excitation, whereas the Z-isomer photoisomerisation involves S2(ππ*) excitation. Conversion between the E and Z isomers through collisional excitation, which is calculated to occur through both inversion and torsion pathways, is investigated experimentally by colliding the molecular ions with nitrogen buffer gas over a range of electric fields. This study demonstrates the versatility of tandem ion mobility mass spectrometry for exploring the isomerisation of molecular photoswitches initiated by either light or collisions.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...