Quantum mechanics/molecular mechanics studies of the mechanism of cysteine protease inhibition by peptidyl-2,3-epoxyketones†
Abstract
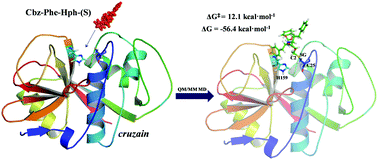
Cysteine proteases are the most abundant proteases in parasitic protozoa and they are essential enzymes to the life cycle of several of them, thus becoming attractive therapeutic targets for the development of new inhibitors. In this paper, a computational study of the inhibition mechanism of cysteine protease by dipeptidyl-2,3-epoxyketone Cbz-Phe-Hph-(S), a recently proposed inhibitor, has been carried out by means of molecular dynamics (MD) simulations with hybrid QM/MM potentials. The computed free energy surfaces of the inhibition mechanism of cysteine proteases by peptidyl epoxyketones showing how the activation of the epoxide ring and the attack of Cys25 on either C2 or C3 atoms take place in a concerted manner. According to our results, the acid species responsible for the protonation of the oxygen atom of the ring would be able to conserve His159, in contrast to previous studies that proposed a water molecule as the activating species. The low activation free energies for the reaction where Cys25 attacks the C2 atom of the epoxide ring (12.1 kcal mol−1) or to the C3 atom (15.4 kcal mol−1), together with the high negative reaction energies suggest that the derivatives of peptidyl-2,3-epoxyketones can be used to develop new potent inhibitors for the treatment of Chagas disease.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...