Effects of CO2 adsorption on proton migration on a hydrated ZrO2 surface: an ab initio molecular dynamics study†
Abstract
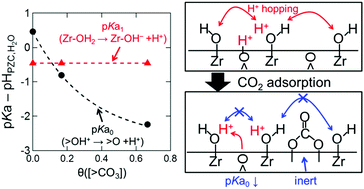
Hydration reactions on a carbonate-terminated cubic ZrO2(110) surface were analyzed using ab initio molecular dynamics (AIMD) simulations. After hydration reactions, carbonates were still present on the surface at 500 K. However, these carbonates are very weak conjugate bases and only act as steric hindrance in proton hopping processes between acidic chemisorbed H2O molecules (Zr–OH2) and monodentate hydroxyl groups (Zr–OH−). Similar to a carbonate-free hydrated surface, Zr–OH2, Zr–OH−, and polydentate hydroxyl groups (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) OH+) were observed, while the ratio of acidic Zr–OH2 was significantly larger than that on the carbonate-free hydrated surface. A thermodynamic discussion and bond property analysis reveal that CO2 adsorption significantly decreases the basicity of surface oxide ions (
OH+) were observed, while the ratio of acidic Zr–OH2 was significantly larger than that on the carbonate-free hydrated surface. A thermodynamic discussion and bond property analysis reveal that CO2 adsorption significantly decreases the basicity of surface oxide ions (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) O), whereas the acidity of Zr–OH2 is not affected. As a result, protons released from
O), whereas the acidity of Zr–OH2 is not affected. As a result, protons released from ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) OH+ react with Zr–OH− to form Zr–OH2, leading to a deficiency of proton acceptor sites, which decreases the proton conductivity by the hopping mechanism.
OH+ react with Zr–OH− to form Zr–OH2, leading to a deficiency of proton acceptor sites, which decreases the proton conductivity by the hopping mechanism.


 Please wait while we load your content...
Please wait while we load your content...