Evaluation of electronic polarization energy in oligoacene molecular crystals using the solvated supermolecular approach†
Abstract
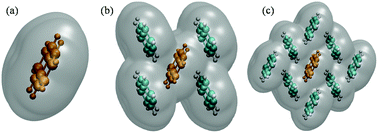
The solvated supermolecular approach, i.e., block-localized wave function coupled with polarizable continuum model (BLW/PCM), was proposed to calculate molecular ionization potential (IP), electron affinity (EA) in the solid phase, and related electronic polarization. Via the calculations of a solvated supermolecule (5M), including four closest molecules, BLW/PCM overcomes the limitation in the calculation for the monomer PCM, that is, nearly same electronic polarization for cation (P+) and anion (P−). The solvated supermolecular approach successfully described asymmetric behaviors of P+ and P− for oligoacene crystals. In addition, we also compared two charge-localized methods, i.e., BLW and constrained density functional theory (CDFT), to calculate the molecular IP and EA in supermolecules with/without PCM. Our results demonstrate that both the BLW and CDFT correctly estimate the EA and IP values in the gas phase cluster, whereas CDFT/PCM fails to evaluate the P− value of the bulk system.


 Please wait while we load your content...
Please wait while we load your content...