Non-zeolitic properties of the dipeptide l-leucyl–l-leucine as a result of the specific nanostructure formation†
Abstract
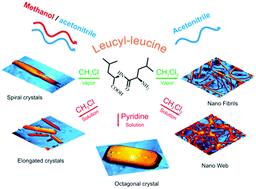
The non-zeolitic behavior of L-leucyl–L-leucine and its self-organization in solid state and from solutions with the formation of different nanostructures are reported. This dipeptide forms porous crystals, but does not exhibit molecular sieve effects typical of classical zeolites and biozeolites. The specific sorption properties of L-leucyl–L-leucine result from a change in its crystal packing from channel-type to layered-type, when binding strong proton acceptors or proton donors of molecular size greater than 18–20 cm3 mol−1. The high sorption capacity of L-leucyl–L-leucine toward dichloromethane results from the self-organization of the dipeptide, by forming nanofibers or web-like structures. The low thermal stability of clathrates of the dipeptide containing large guest molecules and the selectivity of L-leucyl–L-leucine toward alcohols over nitriles can be used to separate organic mixtures such as methanol/n-butanol and methanol/acetonitrile.


 Please wait while we load your content...
Please wait while we load your content...