Time-resolved measurements of product formation in the low-temperature (550–675 K) oxidation of neopentane: a probe to investigate chain-branching mechanism†
Abstract
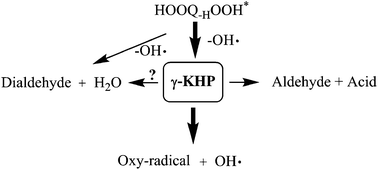
Product formation, in particular ketohydroperoxide formation and decomposition, were investigated in time-resolved, Cl-atom initiated neopentane oxidation experiments in the temperature range 550–675 K using a photoionization time-of-flight mass spectrometer. Ionization light was provided either by Advanced Light Source tunable synchrotron radiation or ∼10.2 eV fixed energy radiation from a H2-discharge lamp. Experiments were performed both at 1–2 atm pressure using a high-pressure reactor and also at ∼9 Torr pressure employing a low-pressure reactor for comparison. Because of the highly symmetric structure of neopentane, ketohydroperoxide signal can be attributed to a 3-hydroperoxy-2,2-dimethylpropanal isomer, i.e. from a γ-ketohydroperoxide (γ-KHP). The photoionization spectra of the γ-KHP measured at low- and high pressures and varying oxygen concentrations agree well with each other, further supporting they originate from the single isomer. Measurements performed in this work also suggest that the “Korcek” mechanism may play an important role in the decomposition of 3-hydroperoxy-2,2-dimethylpropanal, especially at lower temperatures. However, at higher temperatures where γ-KHP decomposition to hydroxyl radical and oxy-radical dominates, oxidation of the oxy-radical yields a new important channel leading to acetone, carbon monoxide, and OH radical. Starting from the initial neopentyl + O2 reaction, this channel releases altogether three OH radicals. A strongly temperature-dependent reaction product is observed at m/z = 100, likely attributable to 2,2-dimethylpropanedial.


 Please wait while we load your content...
Please wait while we load your content...