UV-induced hydrogen-atom transfer and hydrogen-atom detachment in monomeric 7-azaindole isolated in Ar and n-H2 matrices†
Abstract
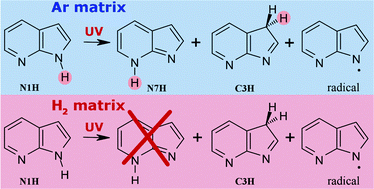
Photochemical transformations were investigated for monomers of 7-azaindole isolated in low-temperature Ar and normal-H2 (n-H2) matrices. The most stable N1H tautomer was the only form of the compound populated in Ar and n-H2 matrices before any irradiation. Upon exposure of Ar matrices to UV (λ > 270 nm) light, two higher-energy tautomers N7H and C3H were photoproduced. Additionally, spectral signatures of the photogenerated 7-azaindolyl radical were also found. All of these photoproducts were experimentally observed for the first time. So far, the N7H tautomer had been known only as a transient species, appearing upon relaxation of photoexcited hydrogen-bonded dimers or complexes. For 7-azaindole isolated in an n-H2 matrix and irradiated at λ > 270 nm, only the C3H tautomer and the 7-azaindolyl radical were photogenerated, whereas the N7H tautomer was not photoproduced at all. The drastic dependence of photogeneration of the N7H form on the matrix environment (solid Ar or solid n-H2) is as a characteristic feature of a specific class of UV-induced hydrogen-atom-transfer processes occurring in matrix-isolated heterocycles.


 Please wait while we load your content...
Please wait while we load your content...