Synergistic effects of plasma–catalyst interactions for CH4 activation†
Abstract
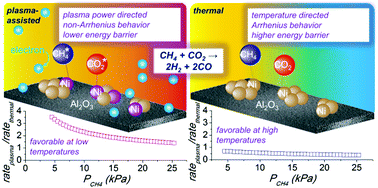
The elucidation of catalyst surface–plasma interactions is a challenging endeavor and therefore requires thorough and rigorous assessment of the reaction dynamics on the catalyst in the plasma environment. The first step in quantifying and defining catalyst–plasma interactions is a detailed kinetic study that can be used to verify appropriate reaction conditions for comparison and to discover any unexpected behavior of plasma-assisted reactions that might prevent direct comparison. In this paper, we provide a kinetic evaluation of CH4 activation in a dielectric barrier discharge plasma in order to quantify plasma–catalyst interactions via kinetic parameters. The dry reforming of CH4 with CO2 was studied as a model reaction using Ni supported on γ-Al2O3 at temperatures of 790–890 K under atmospheric pressure, where the partial pressures of CH4 (or CO2) were varied over a range of ≤25.3 kPa. Reaction performance was monitored by varying gas hourly space velocity, plasma power, bulk gas temperature, and reactant concentration. After correcting for gas-phase plasma reactions, a linear relationship was observed in the log of the measured rate constant with respect to reciprocal power (1/power). Although thermal catalysis displays typical Arrhenius behavior for this reaction, plasma-assisted catalysis occurs from a complex mixture of sources and shows non-Arrhenius behavior. However, an energy barrier was obtained from the relationship between the reaction rate constant and input power to exhibit ≤∼20 kJ mol−1 (compared to ∼70 kJ mol−1 for thermal catalysis). Of additional importance, the energy barriers measured during plasma-assisted catalysis were relatively consistent with respect to variations in total flow rates, types of diluent, or bulk reaction temperature. These experimental results suggest that plasma-generated vibrationally-excited CH4 favorably interacts with Ni sites at elevated temperatures, which helps reduce the energy barrier required to activate CH4 and enhance CH4 reforming rates.


 Please wait while we load your content...
Please wait while we load your content...