Back-exchange: a novel approach to quantifying oxygen diffusion and surface exchange in ambient atmospheres
Abstract
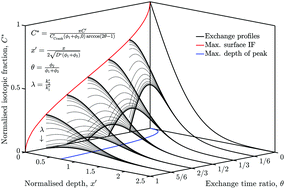
A novel two-step Isotopic Exchange (IE) technique has been developed to investigate the influence of oxygen containing components of ambient air (such as H2O and CO2) on the effective surface exchange coefficient (k*) of a common mixed ionic electronic conductor material. The two step ‘back-exchange’ technique was used to introduce a tracer diffusion profile, which was subsequently measured using Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS). The isotopic fraction of oxygen in a dense sample as a function of distance from the surface, before and after the second exchange step, could then be used to determine the surface exchange coefficient in each atmosphere. A new analytical solution was found to the diffusion equation in a semi-infinite domain with a variable surface exchange boundary, for the special case where D* and k* are constant for all exchange steps. This solution validated the results of a numerical, Crank-Nicolson type finite-difference simulation, which was used to extract the parameters from the experimental data. When modelling electrodes, D* and k* are important input parameters, which significantly impact performance. In this study La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF6428) was investigated and it was found that the rate of exchange was increased by around 250% in ambient air compared to high purity oxygen at the same pO2. The three experiments performed in this study were used to validate the back-exchange approach and show its utility.


 Please wait while we load your content...
Please wait while we load your content...