From oxidative degradation to direct oxidation: size regimes in the consecutive reaction of cationic tantalum clusters with dioxygen†
Abstract
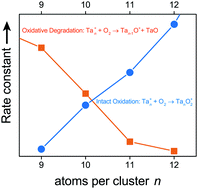
Cationic tantalum clusters (Ta9–12+) are reacted with molecular oxygen under multi-collision conditions in the gas phase in order to analyze the reaction kinetics. Clusters in this transitional size regime demonstrate reaction pathways associated with smaller clusters that mainly degrade upon oxidation (loss of a TaO unit) and those of larger clusters that are oxidized in absence of fragmentation. This behavior is exemplified by Ta9+, which generates Ta9O14+ and Ta4O11+, and the underlying, intricate reaction network is subsequently investigated. Ultimately, rate constants, reaction pathways and final products of cluster sizes up to n = 40 are compared to reveal the size-dependent oxidation behavior.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...