Rare event simulations reveal subtle key steps in aqueous silicate condensation
Abstract
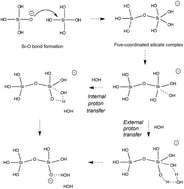
A replica exchange transition interface sampling (RETIS) study combined with Born–Oppenheimer molecular dynamics (BOMD) is used to investigate the dynamics, thermodynamics and the mechanism of the early stages of the silicate condensation process. In this process, two silicate monomers, of which one is an anionic species, form a negatively charged five-coordinated silicate dimer. In a second stage, this dimer can fall apart again, forming the original monomers, or release a water molecule into the solution. We studied the association and dissociation reaction in the gas phase, and the dissociation and water removal step in the aqueous phase. The results on the aqueous phase dissociation suggest two possible mechanisms. The breakage of the bond between the intermediate oxygen and the five-coordinated silicon is sometimes accompanied by a proton transfer. After dissociation into silicate monomers, the anionic monomer is either the previously four-coordinated silicon or the previously five-coordinated silicon depending on whether the hydrogen transfer occurs or not. Our results show that the mechanism of proton transfer is highly predominant. Water removal simulations also show two possible mechanisms distinguished by the proton transfer reaction path. Proton transfer can occur either via a direct or via a water mediated reaction step. The calculations reveal that although both mechanisms contribute to the water removal process, the direct proton transfer is slightly favorable and occurs roughly in six out of ten occasions.

 Please wait while we load your content...
Please wait while we load your content...