Transformation of PbI2, PbBr2 and PbCl2 salts into MAPbBr3 perovskite by halide exchange as an effective method for recombination reduction†
Abstract
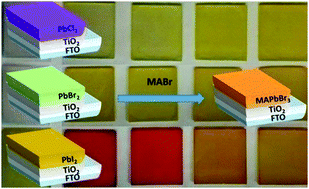
Halide perovskite derivatives present unprecedented physical phenomena among those materials which are suitable for photovoltaics, such as a fast ion diffusion coefficient. In this paper it is reported how the benefits of this property can be used during the growth of halide perovskites in order to control the morphological and optoelectronic properties of the final thin film. Using a large enough halide reservoir, the nature of the halides present in the final perovskite layer can be exchanged and this depends on the initial salt used in the two-step deposition method. In particular, the preparation of a methylammonium lead bromide (MAPbBr3) thin film is reported, using a two-step method based on the transformation of lead(II) iodide (PbI2), lead(II) bromide (PbBr2) and lead(II) chloride (PbCl2) salts into MAPbBr3 perovskite after dipping in a methylammonium bromide (MABr) solution. The films prepared from different salts present different properties in terms of morphology and optoelectronic properties, thus providing significantly different performance when they are used for the preparation of photovoltaic devices. Interestingly, the use of PbI2 and PbCl2 salts reduce the charge recombination and increase the open circuit potential obtained, especially in the former case. However, the highest photocurrent is obtained when PbBr2 is used. For PbI2 and PbCl2 salts no traces of the former salt are observed in the MAPbBr3 layer obtained after 10 minutes of dipping time, however, the presence of PbBr2 has still been detected (using X-ray diffraction) when this salt has been employed.


 Please wait while we load your content...
Please wait while we load your content...