Diels–Alder reactions of graphene oxides: greatly enhanced chemical reactivity by oxygen-containing groups†
Abstract
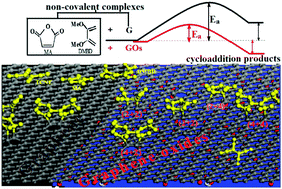
Graphene oxides (GOs) or reduced GOs (rGOs) may offer extraordinary potential for chemical functionalization of graphene due to their unique electronic and structural properties. By means of dispersion-corrected density functional theory computations, we systematically investigated the Diels–Alder (DA) chemistry of GOs. Our computations showed that the dual nature of GOs as both a diene and a dienophile is stronger than that of pristine graphene. Interestingly, the interior bonds of a graphene surface modified by oxygen-containing groups could be functionalized by maleic anhydride (MA) and 2,3-dimethoxybutadiene (DMBD) through cycloaddition reactions, and the cycloaddition products of MA and DMBD are more favorable than the non-covalent complexes between these reagents and the GO surface. The feasibility of covalent functionalization of GOs as a diene and a dienophile strongly depends on the local structural environment of the oxygen groups, including the atomic arrangement and the number of these groups surrounding the reaction site. The exothermicities for (4+2) adducts of DMBD with GO are far larger than those of MA, which indicates that the dienophile character of the GO surface is stronger than its behavior as a diene.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...