Unexpected proton mobility in the bulk phase of cholinium-based ionic liquids: new insights from theoretical calculations
Abstract
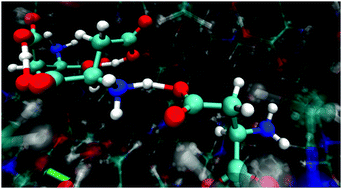
We have explored by means of ab initio molecular dynamics two ionic liquids based on the combination of a choline cation with deprotonated cysteine and aspartic acid anions. While the combination of the strong base choline with various other amino-acids leads to the formation of a highly ionized medium where proton transfer is negligible, the presence of additional protic functions on the SH and COOH groups leads to an unexpected and interesting behavior and to a sizable migration of their acidic protons onto the NH2 basic terminals. As far as we know this is the first time that such proton migration, which in water leads to the well-known zwitterionic form of aminoacids, is observed to take place in their ionized, anionic form. We analyze in detail such dynamical effects using accurate ab initio molecular dynamics computations validated through comparison with X-ray scattering data.


 Please wait while we load your content...
Please wait while we load your content...