Can charged colloidal particles increase the thermoelectric energy conversion efficiency?†
Abstract
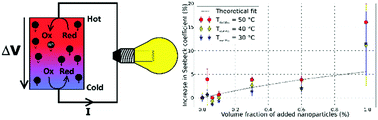
Currently, liquid thermocells are receiving increasing attention as an inexpensive alternative to conventional solid-state thermoelectrics for low-grade waste heat recovery applications. Here we present a novel path to increase the Seebeck coefficient of liquid thermoelectric materials using charged colloidal suspensions; namely, ionically stabilized magnetic nanoparticles (ferrofluids) dispersed in aqueous potassium ferro-/ferri-cyanide electrolytes. The dependency of thermoelectric potential on experimental parameters such as nanoparticle concentration and types of solute ions (lithium citrate and tetrabutylammonium citrate) is examined to reveal the relative contributions from the thermogalvanic potential of redox couples and the entropy of transfer of nanoparticles and ions. The results show that under specific ionic conditions, the inclusion of magnetic nanoparticles can lead to an enhancement of the ferrofluid's initial Seebeck coefficient by 15% (at a nanoparticle volume fraction of ∼1%). Based on these observations, some practical directions are given on which ionic and colloidal parameters to adjust for improving the Seebeck coefficients of liquid thermoelectric materials.


 Please wait while we load your content...
Please wait while we load your content...