On the outside looking in: rethinking the molecular mechanism of 1,3-dipolar cycloadditions from the perspective of bonding evolution theory. The reaction between cyclic nitrones and ethyl acrylate†
Abstract
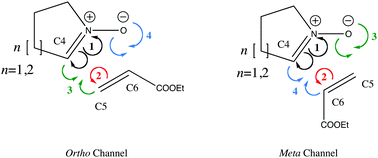
In this work we report on the progress that has been made towards gaining an understanding of the molecular mechanism of 1,3-dipolar cycloadditions using the bonding evolution theory (BET). A detailed analysis of the flow of electron density along the reaction pathway of the formal 1,3-dipolar cycloaddition reaction between cyclic nitrones (pyrroline-1-oxide and 2,3,4,5-tetrahydropyridine-1-oxide) and ethyl acrylate, as a case study, allowed the nature of the molecular mechanisms to be characterized. The present study provides a deep insight into the reaction mechanism, based on the electron density rearrangements given by the structural stability domains, and their connection with the bond breaking/forming processes along the reaction pathway. The electron pushing formalism is a powerful tool to describe chemical reactivity. Here, we show how the Lewis structures can be recovered and how curly arrows describe electron density transfers in chemical reaction mechanisms based on the BET results. The reaction mechanism is described by four consecutive events taking place as the reaction progresses: (1) the population of the initial N–C double bond is transferred to the N and C atoms; (2) the population of the initial double C–C bond is transferred to the C atoms. Along the ortho pathway the next steps are: (3) the C–C bond-forming process, and (4) the O–C bond-forming process. The order of (3) and (4) is inverted in the meta channel. Based on the sequence of the structural stability domains along the intrinsic reaction coordinate, a new synchronicity index is proposed, allowing us to classify and quantify the (a)synchronicity of the 1,3-DC reactions and, therefore, the nature of the reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...