Determination of the diffusion coefficient of hydrogen ion in hydrogels†
Abstract
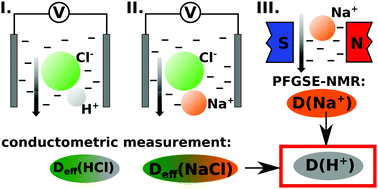
The role of diffusion in chemical pattern formation has been widely studied due to the great diversity of patterns emerging in reaction–diffusion systems, particularly in H+-autocatalytic reactions where hydrogels are applied to avoid convection. A custom-made conductometric cell is designed to measure the effective diffusion coefficient of a pair of strong electrolytes containing sodium ions or hydrogen ions with a common anion. This together with the individual diffusion coefficient for sodium ions, obtained from PFGSE-NMR spectroscopy, allows the determination of the diffusion coefficient of hydrogen ions in hydrogels. Numerical calculations are also performed to study the behavior of a diffusion–migration model describing ionic diffusion in our system. The method we present for one particular case may be extended for various hydrogels and diffusing ions (such as hydroxide) which are relevant e.g. for the development of pH-regulated self-healing mechanisms and hydrogels used for drug delivery.


 Please wait while we load your content...
Please wait while we load your content...