Potential energy surface for the reaction Sm+ + CO2 → SmO+ + CO: guided ion beam and theoretical studies
Abstract
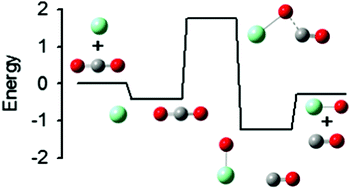
The potential energy surface (PES) for the oxidation of samarium cations by carbon dioxide is explored both experimentally and theoretically. Using guided ion beam tandem mass spectrometry, several reactions are examined as a function of kinetic energy. These include the title reaction as well as its reverse along with the collision-induced dissociation of Sm+(CO2) and OSm+(CO) with Xe. Analysis of the kinetic energy dependent cross sections yields barriers for the forward and reverse oxidation reaction of 1.77 ± 0.11 and 2.04 ± 0.13 eV, respectively, and Sm+–OCO and OSm+–CO bond dissociation energies (BDEs) of 0.42 ± 0.03 and 0.97 ± 0.07 eV, respectively. BDEs for Sm+(CO2)x for x = 2 and 3 are also determined as 0.40 ± 0.13 and 0.48 ± 0.12 eV, respectively. The PESs for the title reaction along the sextet and octet spin surfaces are also examined theoretically at the MP2 and CCSD(T) levels using both effective core potential and all-electron basis sets. Reasonable agreement between theory and experiment is obtained for the experimentally characterized intermediates, although all-electron basis sets and spin–orbit effects are needed for quantitative agreement. The observed barrier for oxidation is shown to likely correspond to the energy of the crossing between surfaces corresponding to the ground state electronic configuration of Sm+ (8F,4f66s1) and an excited surface having two electrons in the valence space (excluding 4f), which are needed to form the strong SmO+ bond.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...