Thermodynamic cycles of the alkali metal–ligand complexes central to electride formation†
Abstract
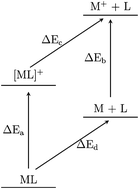
Alkali metal–ligand complexes are the building blocks of the exotic organic alkalide and electride materials. In this work, density-functional theory is used to construct thermodynamic cycles for the alkali metal–ligand complexes, highlighting the energy changes that enable alkalide and electride formation. Strong alkali metal- and cation-to-ligand binding energies are predicted and Rydberg-like ground states of the alkali metal–ligand complexes are identified, consistent with previous work. Calculations on molecular electride species do not reveal consistency with the identified trends, suggesting that the molecular electrides are a class of material unto themselves. The ionisation potentials of the alkali metal–ligand complexes are calculated to be consistently between 1 and 2 eV, suggesting that a specific ionisation potential (IP) is central to electride formation. Further, the thermodynamic cycle for the simplest electride, Cs+(15C5)2e−, shows stabilisation of the solid crystal due to electride formation that is consistent in magnitude with the IP of the equivalent alkali metal–ligand complex. In light of this, computational screening of the alkali metal–ligand complexes' IP presents a new design criterion for alkalide and electride materials.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...