Size and shape effects on the thermodynamic properties of nanoscale volumes of water
Abstract
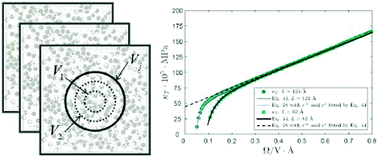
Small systems are known to deviate from the classical thermodynamic description, among other things due to their large surface area to volume ratio compared to corresponding big systems. As a consequence, extensive thermodynamic properties are no longer proportional to the volume, but are instead higher order functions of size and shape. We investigate such functions for second moments of probability distributions of fluctuating properties in the grand-canonical ensemble, focusing specifically on the volume and surface terms of Hadwiger's theorem, explained in Klain, Mathematika, 1995, 42, 329–339. We resolve the shape dependence of the surface term and show, using Hill's nanothermodynamics [Hill, J. Chem. Phys., 1962, 36, 3182], that the surface satisfies the thermodynamics of a flat surface as described by Gibbs [Gibbs, The Scientific Papers of J. Willard Gibbs, Volume 1, Thermodynamics, Ox Bow Press, Woodbridge, Connecticut, 1993]. The Small System Method (SSM), first derived by Schnell et al. [Schnell et al., J. Phys. Chem. B, 2011, 115, 10911], is extended and used to analyze simulation data on small systems of water. We simulate water as an example to illustrate the method, using TIP4P/2005 and other models, and compute the isothermal compressibility and thermodynamic factor. We are able to retrieve the experimental value of the bulk phase compressibility within 2%, and show that the compressibility of nanosized volumes increases by up to a factor of two as the number of molecules in the volume decreases. The value for a tetrahedron, cube, sphere, polygon, etc. can be predicted from the same scaling law, as long as second order effects (nook and corner effects) are negligible. Lastly, we propose a general formula for finite reservoir correction to fluctuations in subvolumes.


 Please wait while we load your content...
Please wait while we load your content...