High level potential energy surface and mechanism of Al(CH3)2OCH3-promoted lactone polymerization: initiation and propagation†
Abstract
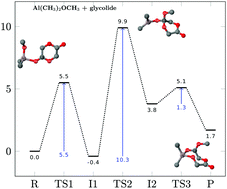
Despite increasing experimental interest in aliphatic polyesters as biodegradable and bioassimilable polymers a theoretical description of ring-opening polymerization (ROP) is not yet fully established. We report a detailed theoretical account of the mechanism of the ROP of three lactones (glycolide, 1,5-dioxepan-2-one and ε-caprolactone) using dimethylaluminium methoxide (Al(CH3)2OCH3) as the initiator. Both the initiation and propagation steps of the ROP are investigated using a composite method consisting of explicitly correlated Moller–Plesset (DF-MP2-F12) and explicitly correlated local coupled cluster methods (DF-LCCSD(T)-F12), for an accurate and definitive determination of the transition state and intermediate electronic energies. A hitherto unreported transition state is found in the initiation reaction, which is the highest energy stationary state for all three lactones. Computed reaction free energies suggest a thermodynamically favourable polymerization of the ROP for all three lactones and a “living mechanism” in the cases of glycolide and 1,5-dioxepan-2-one. The intrinsic reaction coordinate analysis for the ROP of glycolide connects the different stationary states and establishes mechanistic differences between the initiation and propagation reactions. The analysis of structural and electronic parameters along the reaction coordinate reveals a decoupling of structural and electronic changes in the initiation reaction, which allows it to proceed over a lower energy path than in the propagation reaction, where no decoupling is found. Finally, the ab initio electronic energies are compared to popular DFT functionals, where it is found that PBE0 performs best among all tested functionals.


 Please wait while we load your content...
Please wait while we load your content...