Electrolytic conductivity-related radiofrequency heating of aqueous suspensions of nanoparticles for biomedicine†
Abstract
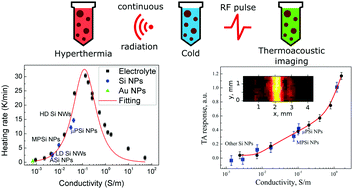
The development of suitable contrast agents can significantly enhance the efficiency of modern imaging and treatment techniques, such as thermoacoustic (TA) tomography and radio-frequency (RF) hyperthermia of cancer. Here, we examine the heating of aqueous suspensions of silicon (Si) and gold (Au) nanoparticles (NPs) under RF irradiation in the MHz frequency range. The heating rate of aqueous suspensions of Si NPs exhibited non-monotonic dependency on the electrical conductivity of the suspension. The experimental results were explained by the mathematical model considering oscillating solvated ions as the main source of Joule heating. These ions could be the product of the dissolution of Si NPs or organic coating of Au NPs. Thus, the ions governed the conductivity of the suspensions, which in turn governs both the heating rate and the near-field RF TA response. The model predicted the contrast in different tissues taking into account both Joule heating and dielectric losses.


 Please wait while we load your content...
Please wait while we load your content...