Insights into the molecular interaction between two polyoxygenated cinnamoylcoumarin derivatives and human serum albumin†
Abstract
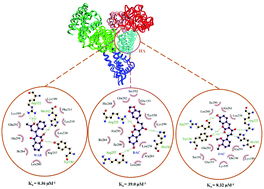
Ligand binding studies on human serum albumin (HSA) are crucial in determining the pharmacological properties of drug candidates. Here, two representatives of coumarin–chalcone hybrids were selected and their binding mechanism was identified via thermodynamics techniques, curve resolution analysis and computational methods at molecular levels. The binding parameters were derived using spectroscopic approaches and the results point to only one pocket located near the Trp214 residue in subdomain IIA of HSA. The protein tertiary structure was altered during ligand binding and formed an intermediate structure to create stronger ligand binding interactions. The best binding mode of the ligand was initially estimated by docking on an ensemble of HSA crystallographic structures and by molecular dynamics (MD) simulations. Per residue interaction energies were calculated over the MD trajectories as well. Reasonable agreement was found between experimental and theoretical results about the nature of binding, which was dominated by hydrogen bonding and van der Waals contributions.


 Please wait while we load your content...
Please wait while we load your content...