Structure of liquid water – a dynamical mixture of tetrahedral and ‘ring-and-chain’ like structures†
Abstract
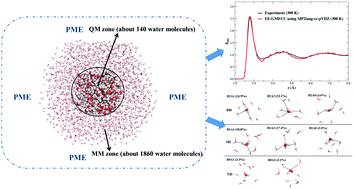
The nature of the dynamical hydrogen-bond network of liquid water under ambient conditions has challenged both experimental and theoretical researchers for decades and remains a topic of intense debate. In this work, we addressed the structural issue of the hydrogen-bond network of liquid water based on an accurate ab initio molecular dynamics simulation. The present work showed clearly that liquid water is neither accurately described by a static picture of mostly tetrahedral water molecules nor dominated by “ring-and-chain” like structures. Instead, the structure of water is a dynamical mixture of tetrahedral and ‘ring-and-chain’ like structures with a slight bias toward the former. On average, each water molecule forms about three hydrogen bonds with the surrounding water molecules. The present accurate ab initio molecular dynamics simulation of liquid water was made possible by using a fragment-based second-order Møller–Plesset perturbation theory (MP2) with a large basis set to treat a large body of water molecules. This level of ab initio theory is sufficiently accurate for describing water interactions, and the simulated structural and dynamical properties of liquid water, including radial distribution functions, diffusion coefficient, dipole moment, etc., are uniformly in excellent agreement with experimental observations.


 Please wait while we load your content...
Please wait while we load your content...