Electronic properties of reduced molybdenum oxides
Abstract
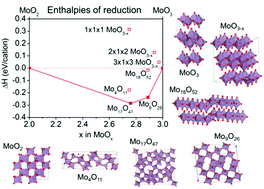
The electronic properties of MoO3 and reduced molybdenum oxide phases are studied by density functional theory (DFT) alongside characterization of mixed phase MoOx films. Molybdenum oxide is utilized in compositions ranging from MoO3 to MoO2 with several intermediary phases. With increasing degree of reduction, the lattice collapses and the layered MoO3 structure is lost. This affects the electronic and optical properties, which range from the wide band gap semiconductor MoO3 to metallic MoO2. DFT is used to determine the stability of the most relevant molybdenum oxide phases, in comparison to oxygen vacancies in the layered MoO3 lattice. The non-layered phases are more stable than the layered MoO3 structure for all oxygen stoichiometries of MoOx studied where 2 ≤ x < 3. Reduction and lattice collapse leads to strong changes in the electronic density of states, especially the filling of the Mo 4d states. The DFT predictions are compared to experimental studies of molybdenum oxide films within the same range of oxygen stoichiometries. We find that whilst MoO2 is easily distinguished from MoO3, intermediate phases and phase mixtures have similar electronic structures. The effect of the different band structures is seen in the electrical conductivity and optical transmittance of the films. Insight into the oxide phase stability ranges and mixtures is not only important for understanding molybdenum oxide films for optoelectronic applications, but is also relevant to other transition metal oxides, such as WO3, which exist in analogous forms.


 Please wait while we load your content...
Please wait while we load your content...