Obtaining effective rate coefficients to describe the decomposition kinetics of the corannulene oxyradical at high temperatures†‡
Abstract
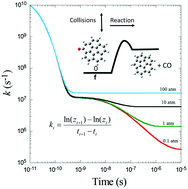
Unimolecular reactions play an important role in combustion kinetics. An important task of reaction kinetic analysis is to obtain the phenomenological rate coefficients for unimolecular reactions based on the master equation approach. In most cases, the eigenvalues of the transition matrix describing collisional internal energy relaxation are of much larger magnitude than and well separated from the chemically significant eigenvalues, so that phenomenological rate coefficients may be unequivocally derived for incorporation in combustion mechanisms. However, when dealing with unimolecular reactions for a large molecule, especially at high temperatures, the large densities of states of the reactant cause the majority of the population distribution to lie at very high energy levels where the microcanonical reaction rate constants are large and the relaxation and chemical eigenvalues overlap, so that well-defined phenomenological rate coefficients cannot be determined. This work attempts to analyze the effect of overlapping eigenvalues on the high-temperature kinetics of a large oxyradical, based on microcanonical reaction rates and population distributions as well as the eigenvalue spectrum of the transition matrix from the master equation. The aim is to provide a pragmatic method for obtaining the most effective rate coefficients for competing elimination, dissociation, and bimolecular reactions for incorporation in combustion mechanisms. Our approach is demonstrated with a representative example, thermal decomposition and H addition reactions of the corannulene oxyradical.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...