Calculations of current densities and aromatic pathways in cyclic porphyrin and isoporphyrin arrays†
Abstract
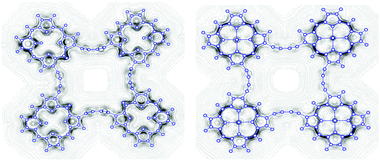
Magnetically induced current density susceptibilities have been studied for a number of cyclic ethyne- and butadiyne-bridged porphyrin and isoporphyrin arrays. The current density susceptibilities have been calculated using the gauge-including magnetically induced current (GIMIC) method, which is interfaced to the TURBOMOLE quantum chemistry code. Aromatic properties and current pathways have been analyzed and discussed by numerical integration of the current density susceptibilities passing selected chemical bonds yielding current strength susceptibilities. Despite the interrupted π-framework, zinc(II) isoporphyrin sustains a ring current of ca. 10 nA T−1. Porphyrin and isoporphyrin dimers sustain a significant current strength at the linker, whereas the larger porphyrinoid arrays sustain mainly local ring currents. Isoporphyrin dimers with saturated meso carbons have strong net diatropic ring-current strengths of 20 nA T−1 fulfilling Hückels aromaticity rule. Porphyrin trimers and tetramers exhibit almost no current strength at the linker. The porphyrin moieties maintain their strong net diatropic ring current.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...