Dimerization and conformation-related free energy landscapes of dye-tagged amyloid-β12–28 linked to FRET experiments†
Abstract
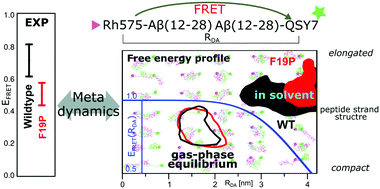
We have investigated the free energy landscape of Aβ-peptide dimer models in connection to gas-phase FRET experiments. We use a FRET-related distance coordinate and one conformation-related coordinate per monomer for accelerated structural exploration with well-tempered metadynamics in solvent and in vacuo. The free energy profiles indicate that FRET under equilibrium conditions should be significantly affected by the de-solvation upon the transfer of ions to the gas-phase. In contrast, a change in the protonation state is found to be less impacting once de-solvated. Comparing F19P and WT alloforms, for which we measure different FRET efficiencies in the gas-phase, we predict only the relevant structural differences in the solution populations, not under gas-phase equilibrium conditions. This finding supports the hypothesis that the gas-phase action-FRET measurement after ESI operates under non-equilibrium conditions, with a memory of the solution conditions – even for the dimer of this relatively short peptide. The structural differences in solution are rationalized in terms of conformational propensities around residue 19, which show a transition to a poly-proline type of pattern upon mutation to F19P – a difference that gets lost in the gas-phase.


 Please wait while we load your content...
Please wait while we load your content...